
Copyrights(c) 2005-2011, Dr. R. R. Stiffler
Spatial Energy
Coherence
Copyrights(c)
2005-2011, Dr. R. R. Stiffler
|
DO NOT attempt
replication of my research, unless you are fully aware of
the dangers involved in working with Hydrogen gas. You must
insure that the Hydrogen and Oxygen are not allowed to mix.
Mixtures of H2 and O2 are reactive in concentrations from
~4% > 74%. All apparatus should be vaccum tested and evacuated
of Oxygen before Hydrogen production is commenced. Stiffler
Scientific is not responsible for, or holds any
libility for damage or injury to person or property resulting
from the duplication of our work. Additionally I performed a number of experiments with a somewhat controversial Pulsed cell arrangement using a low voltage pulsed power supply. The pulsed power method I am referring to is attributed to a Stanley Meyer (somewhat controversial individual, do Internet search for further information on Stanley Meyer) that developed a circuit using high voltage pulsed power. Not wanting to duplicate the work of Meyer, I constructed a switching (pulsed) power supply running at 12 volts DC that was switched into a two plate electrolyzer cell. The switching rate was adjustable and the circuit itself was a rather simple circuit overall. Initial testing did not produce surprising or unexpected results. The cells action and output performed as expected and calculated. A number of
experiments were conducted with resonant circuits (both
parallel and series) in the cells return power leg (anode).
A number of configurations were tested before even the slightest
indication of something different was observed. A slight
oscillation in the circuit and a visible increase (although
small) in gas production, without seeing increased input was
the first indication of something different in the test
runs. What was different was this particular circuit had a
feedback path from the resonant components back to the cathode
side of the cell. Gas production increased significantly while maintaining the same input to the cell. Something was taking place in this particular circuit that indicated a generation efficiency of over 100%, but appeared to be outside of any direct molecular interaction.. I constructed a large cell, one that would allow for a large volume of gas so that exact output measurements could be performed and appropriate calculations performed. Because of a mistake in staying with the Rhodes(a) gas mixture I set the stage for what was to be a near tragic yet avoidable accident. Stating again that the Rhodes(a) gas, an evolved combination of Hydrogen and Oxygen in my opinion is extremely dangerous and should be avoided without exception. (This gas mixture and its apparent dangers arise from the storage of the gas, rather than the generation and real time usage of the gas, provided safety standards are carefully followed.) With an improved design while storing close to a liter of this highly volatile mixture, it happened. Some how the mixture became catalyzed and Bang!. The entire cell was blown apart with the lid and hose fixtures embedding into the soft lab ceiling. Pieces of the cell were found some 10 meters away from where the explosion took place. The hose fitting on the lid to the cell pushed one half inch into the ceiling tile. Further testing was stopped while consideration was given to the hazards involved in this type of research being conducted in a general research lab. In mid 2004
after reading numerous articles on the direction alternate energy
production was taking, I again decide to resume
research into real time hydrogen fuel production. This time I
chose to use separation cells (dual duct) and implement
strict production, storage and handling procedures. I began with a common or standard cell design composed of anode and cathode electrodes (1/4" carbon rods) without membrane separation, yet fully separating the H2 from the O2. Pressure adjustments and balancing were not of initial concern. Once establishing a base line for comparative measurements I looked into the design of the electrodes and what if any impact different geometry had on overall production efficiency. Initial research results were text book, output was without a doubt a function of input, even though I did see some minor increases in production efficiencies from different electrode designs, shape and feed points. Electrodes One of my early circuits contained a resonant circuit, a few capacitors and some high speed switching diodes. Scope traces and pulse timing measurements indicated that the resonant circuit was not a contributor to the process owing to its resonance. Rather it was acting as a time delay for pulses being reapplied to the input of the cell. Once this was understood I began to form a conceptual picture of how the CRE was working. When reading this paper, one must keep in mind that for proper disassociation of H2 and O2 through electrolysis that the electron conduction is ionic, not resistive in the conventional sense. This fact is significant in the understanding and operation of the CRE. The CRE supplies electrode voltages of from 2 volts to 4 volts, depending on the composition of the water and electrolyte. The more conductive the water the higher the applied electrode voltage will be. Let me offer an explanatory mind picture of how CRE is working. Picture a fire brigade using buckets with a central supply of water some distance away from a particular fire. To fight the fire each man runs to the water supply, fills his bucket, runs back to the fire and disperses the water over the fire. The men must then run back to the water supply for the next bucket of water and keep repeating the process until the fire is out. In this process each man expends X amount of energy for each bucket of water he obtains and throws upon the fire. One
day a rather tired fire fighter noted that not all of the water
thrown onto a fire was evaporated from the fires heat and
some water ran off and pooled in a ditch a few feet from the
fire. The fire fighter reasoned that one bucket of water was just
as good as the next so he saved the long run to the supply pool
and drew his buckets from the closer source of the ditch, thereby
recycling some of the water while expending less of his own
energy. When the fire was finally out, our industrious fire
fighter that took water from the ditch used X' energy during
the event, while the rest of the fire fighters each used X
energy. Our fire fighter saved (X - X')energy by recycling the
water. So how does this apply to the CRE? Simple, the water is replaced by electrons from the power source to the electrolyzer and the CRE is the smart fire fighter that uses some of those electrons over in the process of the disassociation of the H2 and O2. As far as the power source is concerned it cares less how many times a particular electron is used before being returned to the anode. Neither does the power source care how long it really takes for a particular electron to travel from the cathode back to the anode, provided it does return. I may need to clear up a possible connection between energy and efficiency that could lead to a misunderstanding. Assume a standard two plate electrolyzer cell that is capable of producing 1 liter of H2 per hour with an electrical input of ( Xb Joules) at an efficiency of 80%. This same cell under the control of a CRE controller could easily produce 1.8 liters per hour with the same input of (Xb Joules). The additional energy comes from the CRE controller recycling electrons back through the cell for a second trip. The cell efficiency has crossed the magic 100% barrier because I are now disassociating additional gas over and above that which would be produced by the source (Xb Joules) alone. The increased production cannot exceed 200%, limited by the fact that I can only use a single electron over one time. Current test CRE's run comfortably around 80% to 91% over source input. Consider the following two reactions that take place at the cell electrodes. Cathode:
4H2O + 4e- => 4OH- + 2H2
In its simplicity the CRE is a circuit that recycles electrons and does not violate thermodynamics in the application of this method in the disassociation of the H2 and O2 gases. The CRE will not work for recycling of electrons in a purely resistive load as the entropy will prevent an efficiency gain. It must be mentioned again that electrolysis does not depend on the generation of heat and therefore is not subject to the thermodynamic limitation on efficiency. The switching circuits in the CRE use energy supplied by the power source, which lowers overall efficiencies by from 10%-20%, although new designs using MOSFETs and Opto Switches can limit this loss to below 20%. Current test CRE's are using a switch controlled by a CMOS 555 adjustable timer that allows a maximum of 191% effective gain. In a production cell I feel the effectiveness will be in the range of 170% to 180% in order to keep circuit complexity and module cost low. 03/11/2006 Current CRE switches now use four MOSFET's with two in a multi-vibrator circuit and the remaining two with two high speed, high current switching diodes form the capacitor switch. The new arrangement allows for the timing and switch components (not including capacitor bank) to be priced at less than $5 (US). For a high volume CRE , the cost of the capacitors can run into the hundreds of dollars and require that additional MOSFET switches be used. The CRE will work on conventional and PEM cells although with the PEMs a 2W output cell remains a 2W cell because its production limitations are more or less built into the cell itself. Although you would with the CRE be able to max out the PEM production limit with less input power than is normally be required. CRE circuit
diagram? Not shown are the required switching components. When
the power source is initially connected the electron pressure at
the cathode supplies an electron to an H+ ion. (H+ although not
truly correct is used for illustration.) While at the anode a HO-
ion is giving up an electron. During this continued process the
H2(g) and O2(g) are produced. The electrons flowing through the
cell and causing the Redox reaction are also storing energy
in capacitor C1. When C1 has reached 1T, the switching circuit
disconnects the power source and connects C1 across the cell. C1
dumps its stored energy in the form of recycled electrons back
into the cell to continue the Redox reaction. The switching
circuit keeps switching back and forth between the power source
and C1. The following
is a picture of a working test model CRE. The follow circuit indicates the construction of a low current solid state CRE driver. Using a solid state driver removes the draw back of the mechanical relay which would only offer limited service do to the high number of contact closures per minute that would be required in a fully operational system. There is a waste of power in the solid state unit and that is the dissipation of diodes D1 and D2. With 1A of current each will dissipate 0.7 watts. D1 and D2 are used although in that they reduce the complexity of the overall switching circuit and reduce cost.
12/15/2006 To illustrate what a CRE looks like in operation I set up a simple demonstration with two carbon electrodes in a lab beaker, driven by a CRE. As you will see in the video this is not a separation cell and is releasing all gases into the open air. What is important is looking at the cathode or H2 electrode on the left side of the picture. You will observe bursts of gas as the CRE pulses. Each alternate burst is charge recycled during the preceding power cycle. Remember that CRE disconnects the power source from the cell for 50% of the time. The remaining time, recycled charge is flowing through the electrolyte. This video is MPEG
and 600k in size. Once the input current decreases, production also decreases and overall decrease in efficiency. This decrease is easily overcome by the addition of a second electrode. In a three electrode cell system the electrical anode is maintained at +V, while the two electrical cathodes are alternately switched to -V with the duty cycle of 50% alloted to each electrode. What will be seen is that the cell will maintain its peak input current and overall efficiency will be much improved. What aids in the increase of production is not obvious at first, yet is a mix of jarring bubbles from the electrodes and a fracturing of the ion cloud around each electrode (a form of directed mixing). During the switch period, one electrode sees a reversal in polarity that tends to push the attached gas bubbles from the electrode surface as well as away from the electrode. At the same time the other electrode is moving upward in voltage in the correct polarity. The following
image is a simple diagram of this type of switch circuit. 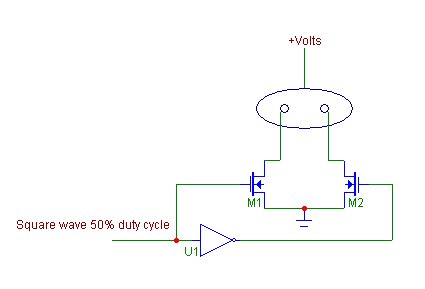
12/15/2006
Care is required so that the switch frequency is kept as low as possible to obtain the increase in efficiency, yet not increase the cell heat by a significant amount. Because the molecules are shifting direction there will be additional collisions and heat. Strangely enough the cell seems to learn what is taking place and the additional heat will decrease over a period of time. This decrease can be observed if the switch frequency is adjusted slowly upward. There will be a point where the heat will continue to rise with no fall back. The optimal frequency is of course below this level. It should be obvious that you can not just place two electrodes in a cell with a switching circuit and obtain optimal results. The entire cell must be taken into consideration in the design and the ability to determine the electrode size and spacing from each other as well as the spacing of each from the electrical anode. Pure water or electrolyte also factors into the equation. At his time the number of variables involved in various cell parameters place this design into the art class leaning heavenly on empirical data from a limited number of controlled cells. The information is being provided as presented, informational only.
The following pictures are of the bubblers, scrubbers (or dryers) and flame traps (flame arrestors). I use three types of flame arrestors, bubblers, water filled U tubes and silicate sand supported by screens and fiberglass cloth. The following is a picture of a completed silicate sand arrestor . H2 capture and pressure tank in leak testing The electrolyzer
generator cells used for laboratory testing Full Unit Test, All Ready to go First Complete Test a Success (1) First Complete Test a Succes (2) Having hydrogen at half the cost still presents numerous problems, especially for the domestic user, just how will this cheaper hydrogen be used? Feeding hydrogen back into a fuel cell to obtain electricity, makes little sense, you end up paying more overall than just using the utility electricity and forgetting the hydrogen. One must factor in the cost of the electrolyzer, the cost of the fuel cell, water and all the maintenance that would be required. Currently fuel cells just are not efficient enough to make this a working configuration. References:aDr.William A. Rodes. bProf. Yull Brown |